2012 Annual Science Report
 University of Wisconsin
Reporting | SEP 2011 – AUG 2012
University of Wisconsin
Reporting | SEP 2011 – AUG 2012
Project 2F: Potential for Lithotrophic Microbial Oxidation of Fe(II) in Basalt Glass
Project Summary
Ferrous iron (Fe(II)) can serve as an energy source for a wide variety of chemolithotrophic microorganisms (organisms that gain energy from metabolism of inorganic compounds). Fe(II) oxidation may have played a role in past (and possibly, present) life on Mars, whose crust is rich in primary Fe(II)-bearing silicate minerals, as well as Fe-bearing clay minerals formed during weathering of primary silicates. This project examined the potential for microbial oxidation of Fe(II) in basaltic glass. Recent research suggests that near‐surface hydrothermal venting may have occurred during past periods of active volcanic/tectonic activity on Mars. Such activities could have produced basalt glass phases that might have served as energy sources for chemolithotrophic microbial activity. Previous and ongoing NAI‐supported studies have shown that an established chemolithoautotrophic Fe(II)‐oxidizing, nitrate‐reducing culture can grow by oxidation of Fe(II) insoluble Fe(II)‐bearing phyllosilicate phases such as biotite and smectite. The initial goal of this project was to determine whether or not this culture is capable of oxidizing Fe(II) in basalt glass. In addition we tested basaltic glass oxidation by a culture of Desulfitobacterium frappieri, as previous studies demonstrated that D. frappieri is capable of nitrate-dependent oxidation of structural Fe(II) in smectite. Finally, in situ and enrichment culturing experiments were conducted to determine whether indigenous Fe(II)-oxidizing organisms in a groundwater iron seep were capable of colonization and oxidation of basaltic glass. The results of these experiments showed that while the various cultures were readily capable of smectite oxidation with nitrate, none were able to carry-out significant oxidation of Fe(II) in basalt glass. We speculate that Fe(II) atoms in the amorphous glass are somehow occluded and therefore not accessible to outer membrane cytochrome systems thought to be involved in extracellular Fe(II) oxidation.
Project Progress
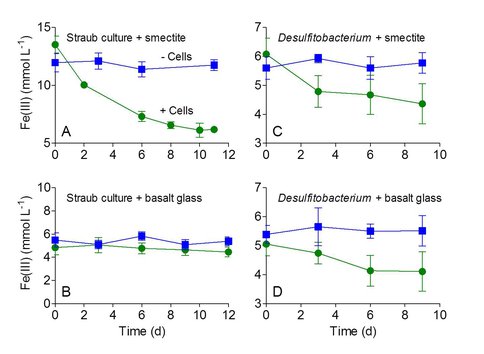
The initial goal of this project was to determine whether an established chemolithoautotrophic Fe(II)‐oxidizing, nitrate‐reducing culture (Blöthe and Roden, 2009; Straub et al., 1996; Weber et al., 2001) is capable of oxidizing Fe(II) in basaltic glass. Previous and ongoing NAI‐supported studies have demonstrated that this culture (referred to as the “Straub culture”) is capable of growing with insoluble Fe(II)‐bearing phyllosilicate phases such as biotite as an energy source (Shelobolina et al., 2012). In addition, prior preliminary studies suggested that this culture is able to oxidize Fe(II) in basalt glass (Honma and Roden, 2010). In contrast to these early results, the new experiments indicated that although the Straub culture could readily able to oxidize Fe(II) in reduced smectite (a secondary Fe-phyllosilicate mineral), Fe(II) in fresh, finely ground basalt glass (obtained from an active lava flow from the Kilauea volcano in Hawaii) was not susceptible to oxidation (Figure 1A,B).
Figure 1. Oxidation of Fe(II) in smectite and basalt glass by the Straub culture (A,B) or Desulfitobacterium frappieri (C,D) in comparison to sterile, uninoculated controls. The results show the mean +/- standard deviation of triplicate cultures.
In contrast, Desulfitobacterium frappieri, which was shown previously to be capable of nitrate-driven oxidation of structural Fe(II) in smectite (Shelobolina et al., 2003), was able to oxidize Fe(II) in basalt glass (Figure 1C,D). Unlike the Straub culture, D. frappieri does not grow chemolithoautotrophically (i.e. it requires a carbon source and nutritional supplements for growth), and is known to solubilize a substantial amount of Fe from smectite (Shelobolina et al., 2003). These results suggests that Fe in basalt glass must be solubilized to facilitate enzymatic oxidation, which prior experiments (Shelobolina et al., 2012) indicate is not the case for the chemolithoautotrophic Straub culture.
As a follow-up to the above experiments with defined microbial cultures, enrichment culturing experiments were conducted to determine whether indigenous Fe(II)-oxidizing organisms in a groundwater iron seep (located on Nature Conservancy lands ca. 50 km north of Madison, WI) were able to oxidize Fe(II) in basaltic glass.
Figure 2. The groundwater Fe seep site in Hemlock Draw Preserve, WI.
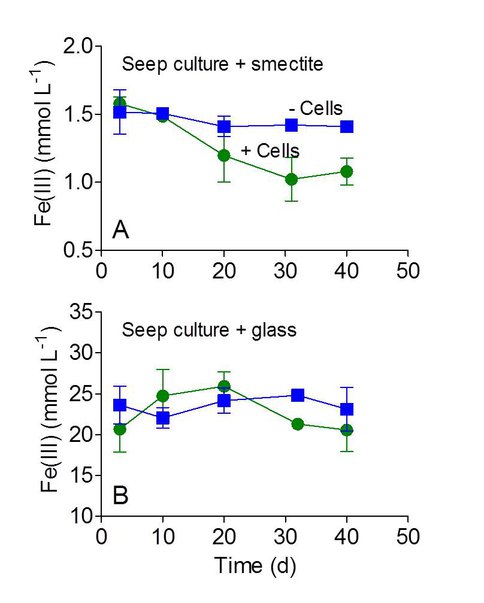
Although groundwater seeps are not direct analogs to volcanic terrain environments that can support Fe-based microbial ecosystems, they contain a wide variety of Fe(II)-oxidizing organisms that could serve as models for potential modes of Fe(II) oxidation activity. The results of these experiments showed that while an enrichment culture derived from the seep materials was readily capable of smectite oxidation with nitrate, it was unable to carry-out significant oxidation of Fe(II) in basalt glass.
Figure 3. Oxidation of Fe(II) in smectite and basalt glass by groundwater seep enrichment culture in comparison to sterile, uninoculated controls. The results show the mean +/- standard deviation of triplicate cultures.
Based on these results, we speculate that Fe(II) atoms in the amorphous glass are somehow occluded and therefore – in the absence of mineral dissolution – not accessible to outer membrane cytochrome systems thought to be involved in extracellular Fe(II) oxidation. These results will be confirmed in future experiments with other novel chemolithotrophic Fe(II)-oxidizing organisms known to be capable of direct oxidation of insoluble Fe(II)-bearing phyllosilicates.
References
Blöthe, M., Roden, E.E., 2009. Composition and activity of an autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture. Appl. Environ. Microbiol. 75, 6937–6940.
Honma, A., Roden, E.E., 2010. Chemolithotrophic microbial oxidation of basalt glass. Astrobiology Science Conference 2010, Abstract 5367.
Shelobolina, E.S., Gaw-VanPraagh, C., Lovley, D.R., 2003. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol. J. 20, 143-156.
Shelobolina, E.S., Xu, H., Konishi, H., Kukkadapu, R., Wu, T., Blothe, M., Roden, E.E., 2012. Microbial lithotrophic oxidation of structural Fe(II) in biotite. Appl. Environ. Microbiol. 78, 5746–5752.
Straub, K.L., Benz, M., Schink, B., Widdel, F., 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Environ. Microbiol. 62, 1458-1460.
Weber, K.A., Picardal, F.W., Roden, E.E., 2001. Microbially-catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ. Sci. Technol. 35, 1644-1650.
Publications
-
Shelobolina, E., Xu, H., Konishi, H., Kukkadapu, R., Wu, T., Blothe, M., & Roden, E. (2012). Microbial Lithotrophic Oxidation of Structural Fe(II) in Biotite. Applied and Environmental Microbiology, 78(16), 5746–5752. doi:10.1128/aem.01034-12
- Honma, A. & Roden, E.E. (2010). Chemolithotrophic microbial oxidation of basalt glass. Astrobiology Science Conference 2010, Abstract 5367.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Evgenya Shelobolina
Research Staff
Mai Yia Xiong
Graduate Student
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments