2009 Annual Science Report
 University of Wisconsin
Reporting | JUL 2008 – AUG 2009
University of Wisconsin
Reporting | JUL 2008 – AUG 2009
New Frontiers in Micro-Analysis of Isotopic Compositions of Natural Materials: Development of Fe Isotopes
Project Summary
We are developing micro-analytical techniques to perform in situ Fe isotope analysis of Fe-bearing minerals by ion microprobe and laser ablation mass spectrometry. Iron isotope compositions are important signatures in tracking redox processes, chemical weathering, and dissimilatory iron reduction by bacteria. In situ micro analysis procedures will allow us to better apply the Fe isotope system by allowing one to determine Fe isotope compositions within a petrographic framework, minimize sample requirements, evaluate microscale heterogeneity, and inter-mineral isotopic equilibrium. Such in situ procedures are critical for analysis of samples that may be returned from future space missions or for analysis by instruments that can be deployed on space craft.
Project Progress
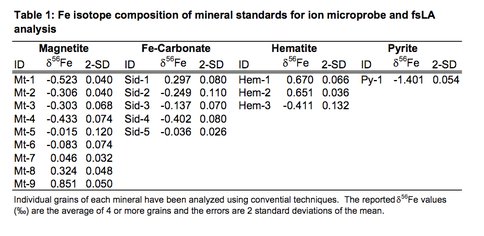
We are evaluating the capabilities of the ion microprobe and femtosecond laser ablation (fsLA) for Fe isotope analysis of different Fe-bearing minerals. This work has focused on using the WiscSIMS facility and the newly installed fsLA system in the UW Radiogenic Isotope laboratory to determine the best instrument operating conditions. In parallel with this work, different Fe-bearing minerals are being evaluated for suitability of a Fe isotope standard. To date we have developed a series of Fe isotope mineral standards including magnetite, hematite, pyrite, and Fe carbonate (Table 1). This work has entailed back-scattered electron imaging and electron microprobe wavelength-dispersive analysis to determine the extent of chemical zonation, and major element composition.
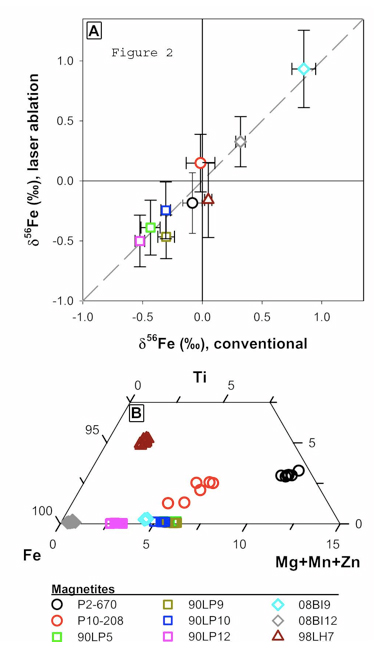
Figure 2 A. Figure 2 A: Plot of Fe isotope composition of different magnetite standards measured by fsLA versus their Fe isotope composition determined using solutions. Error bars are 2-sigma. Figure 2B: Ternary diagram of magnetite compositions as determined by electron microprobe.
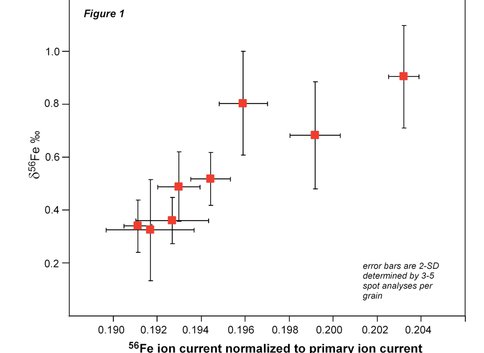
We have established the best running conditions for magnetite analysis by ion microprobe. With a 25 micron sized O- primary beam, the 56Fe/54Fe ratio can be measured to an internal 2-standard error precision of 0.15 ‰ based on 4 minutes of sputtering time. Multiple analyses of a single mineral grain indicate that overall external reproducibility (2-standard deviations) is the same. However, multiple analyses of magnetite grains that are homogenous to +0.050 ‰ define a 0.6 ‰ of δ56Fe values which are positively correlated with the ion yield (56Fe ion current normalized to primary ion beam current) (Figure 1). This variability in δ56Fe values in a chemically and isotopically homogenous material is probably the result of crystal orientation effects associated with ion channeling and implanting of O2 into the crystal structure resulting in relative changes in ion yield (Kita et al., 2010). We are currently investigating methods to minimize fractionation by using different energy offsets and primary acceleration potentials as well as developing methods to correct for crystal orientation mass bias effects based the crystallographic orientation of the mineral. We are also investigating related effects for oxygen and sulfur isotopes in magnetite and sphalerite (see project report: New frontiers in micro-analysis of isotopic compositions of natural materials: Development of O, S, Si, and Li isotopes). We highlight that this crystal orientation effect is not expected for phases such as pyrite or Fe carbonate and thus these phases may be more amenable to analysis by ion probe.
Table 1.
We are in the initial stages of developing methods for Fe isotope analysis using fsLA coupled to multi-collection inductively coupled plasma mass spectrometry (MC-ICP-MS). So far, we have focused on magnetite because this is the most important oxide mineral in banded iron formations, as well as early Archean black shales. Preliminary data indicates that the fsLA is free of matrix effects associated with sample heating as well as chemical differences in the phase (Figure 2), confirming the promise that ultra-fast laser pulses of ~100 fs (10-13 s) have clear advantages as compared to traditional nanosecond (10-9 s) lasers. For example, magnetite analyses are accurate over a wide range of magnetite compositions (Fig. 2A) that range from nearly endmember magnetite to samples with high concentrations of Ti, Mg, Mn and Zn (Fig. 2B). The internal (2-SE) and external precision (2-SD) of each analysis is ~0.15 ‰ in 56Fe/54Fe, which, remarkably, is only slightly poorer than that obtained using solutions. Laser spot sizes ranged from 15 and 20 microns and the sample was scanned back and forth (~5 passes) over a 75 micron linear segment. An analysis lasts 400 seconds and is a preceded by a 60 second gas blank analysis which is subtracted from the on-peak analyte measurement. These results demonstrate that fsLA, coupled to MC-ICP-MS promises to revolutionize in situ isotopic analysis of geologic materials for elements amenable to MC-ICP-MS analysis. In contrast, elements not readily analyzed using MC-ICP-MS, such as O and C, will likely remain in the realm of ion probe analysis.
Figure 1. Figure 1: The measured Fe isotope composition of different grains of magnetite standard 8 as determined by ion microprobe. The positive correlation between Fe isotope composition and ion yield reflects mineralogic orientation of the different magnetite grains
Publications
- Kita, N., Huberty, J.M., Kozdon, R., Beard, B.L. & Valley, J.W. (2010, In Press). High precision SIMS oxygen, sulfur, and iron stable isotope analyses of geological materials: Accuracy, surface topography, and crystal orientation. SIMS XVII Proceedings special issue Surface and Interface Analysis.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Clark Johnson
Co-Investigator
Noriko Kita
Co-Investigator
John Valley
Co-Investigator
Andrew Czaja
Postdoc
Philipp Heck
Postdoc
Reinhard Kozdon
Postdoc
Weiqiang Li
Postdoc
John Fournelle
Research Staff
Brian Hess
Research Staff
Jim Kern
Research Staff
Mike Spicuzza
Research Staff
Jason Huberty
Graduate Student
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems