2009 Annual Science Report
 Montana State University
Reporting | JUL 2008 – AUG 2009
Montana State University
Reporting | JUL 2008 – AUG 2009
Biomimetic Cluster Synthesis: Bridging the Structure and Reactivity of Biotic and Abiotic Iron-Sulfur Motifs
Project Summary
Synthetic approaches are being utilized to bridge the gap between Fe-S minerals and highly evolved biological Fe-S metalloenzymes. These studies are focusing on organic template (protein) mediated cluster assembly (biomineralization), probing properties of synthetic clusters, both as homogeneous and heterogeneous catalysts, investigating the impact of size scale on the properties of synthetic Fe-S clusters, and computational modeling of the structure and catalytic properties of synthetic Fe-S nanoparticles in the 5-50 nm range.
Project Progress
Efforts during the last year have focused primarily on the determination of the structure of iron-sulfide nanoparticles synthesized in our laboratory using protein-cage assisted protocols. Our experimental technique of choice has been Pair Distribution Function (PDF) analysis – a method in which high-energy X-ray scattering is collected and Fourier transformed to obtain the real-space atomic correlations or PDF. In this approach, the function G(r) gives the probability of finding an atom at a distance r from an average atom within the structure; peaks in G(r) correspond to atom-atom distances in a crystalline lattice. This data can then be tested against various models of material structure. Experiments conducted at the Advanced Photon Source at Argonne National Laboratories have indicated that the protein-templated FeS particles form a distorted mackinawite structure with an extremely small crystalline domain size (< 1nm). Significant amounts of Fe oxide are still present (depending on preparation conditions), and the FeS probably forms a layer around an oxide core. Samples were prepared under a variety of pH conditions and at different Fe/cage loading ratios; these parameters appear to have had fairly little effect on the structure as determined by PDF.
Work has also been done on measuring hydrogen evolution from the FeS nanoparticles using gas chromatography. The assumption was that if the disordered FeS layer contains defects that are similar in structure to a hydrogenase active site, some H2 evolution should be observed. Reaction conditions used methyl viologen as an electron carrier and dithionite as a sacrificial electron donor. H2 evolution experiments were performed both on the FeS nanoparticles described above and on particles that had been doped with 7% Ni. In both cases, no hydrogen evolution was observed.
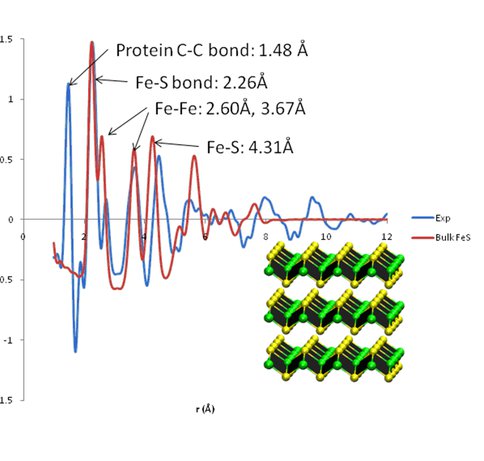
PDF of FeS particle inside of PfFn cage, compared with calculated PDF for mackinawite with 8Å crystalline domains. The 1.5Å C-C covalent bond length from the protein cage is visible in the experimental PDF but absent in the calculated mineral PDF. The Fe-S bond length is in excellent agreement, while other distances appear to be somewhat longer than in the crystal structure, consistent with a distorted mackinawite structure. The 4.15Å distance corresponding to the inter-layer spacing is decidedly absent in the experimental PDF, suggesting that the layered structure of bulk mackinawite is not present in our protein-templated nanoparticles.
Publications
-
Klem, M. T., Young, M., & Douglas, T. (2010). Biomimetic synthesis of photoactive α-Fe 2 O 3 templated by the hyperthermophilic ferritin from Pyrococus furiosus. J. Mater. Chem., 20(1), 65–67. doi:10.1039/b918620d
- Klem, M., Young, M. & Douglas, T. (2008). Biomimetic synthesis of b -TiO 2 inside a viral capsid. J. Materials Chem, 18: 3821-3823.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
John Parise
Co-Investigator
Daniel Strongin
Co-Investigator
Mark Young
Co-Investigator
Richard Harrington
Postdoc
Craig Jolley
Postdoc
Riley Murphy
Doctoral Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 3.4
Origins of cellularity and protobiological systems
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems