2009 Annual Science Report
 Carnegie Institution of Washington
Reporting | JUL 2008 – AUG 2009
Carnegie Institution of Washington
Reporting | JUL 2008 – AUG 2009
Project 4: Geochemical Steps Leading to the Origins of Life
Project Summary
The project titled “Geochemical Steps Leading to the Origins of Life” sets a out a research object focusing on exploring the natural intersection of abiological organic chemistry and the mineral world. Assuming that life emerged on Earth as a consequence of natural, geochemical, processes. We ask what did the organic landscape look like before life, how did organic-mineral surface interactions affect this landscape, and can we identify any connections between this abiotic organic Earth and the subsequent emergence of life.
Project Progress
Task 4.1 Geochemical Roots of Life
Over the past year PI Cody and collaborators have continued their research focusing on network topology of small molecule reactions that start with the reduction of CO2 over transition metal sulfide minerals and lead towards progressively more complex organic structures. Our previous work has shown how many of the familiar metabolic intermediates are readily synthesized through metal sulfide catalyzed carbonyl insertion reactions. Progress in this research has lead to our identifying several potentially important reaction junctions. In one case, we observe the formation of copious amounts of succinic acid from an apparent retroaldol reaction of methylsuccinyl alcohol to succinic acid and formaldedyde. A source of formaldedyde under hydrothermal conditions is significant, due to the fact that formaldehyde is not formed via direct reduction of CO2 under hydrothermal conditions. The presence of formaldehyde and other carbonyl bearing compounds in the reaction mixture suggests that over time, in a continuous reactor, one would expect to build up a complex organic macromolecular phase derived from the formose condensation reaction. Over the past year we begun to explore the molecular structure of the formose polymer using solid state NMR to investigate how such a polymer may have had utility to abiological reactions. We have found that the formose polymer contains considerable olefinic carbon, enenone, and keto/aldehyde groups. Current work is focused on how the presence of such a polymer in the prebiotic world may have been beneficial to the origin of life.
A second component of last year’s research involves the study of surface enhanced peptidization reactions, e.g., increasing the yield of diglycine, triglycine, ...etc. above that which is expected based on solution thermodynamic considerations. In addition to investigating the activity of specific metal sulfide catalysts; we are also focusing on phosphatic silicate glasses as are known to exist on the ocean sea-floor in proximity to deep sea hydrothermal vents associated with spreading centers. This work is on-going and is yielding favorable and in some cases very surprising results. Overall two manuscripts are in preparation and we anticipating submitting these before the end of this year. The results of this research have been presented in invited talks at the ESF High Level Conference on Systems Chemistry, Maratea, Italy, The Department of Environmental and Earth Sciences, Lehigh University, and the Astrobiology Seminar held at the University of Washington, Seattle.
Task 4.2 Prebiotic Molecular Selection
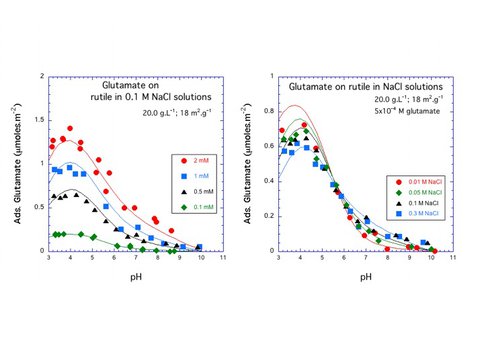
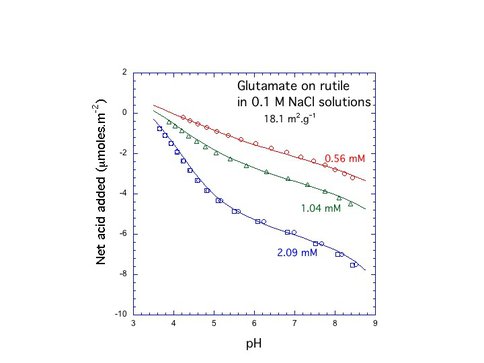
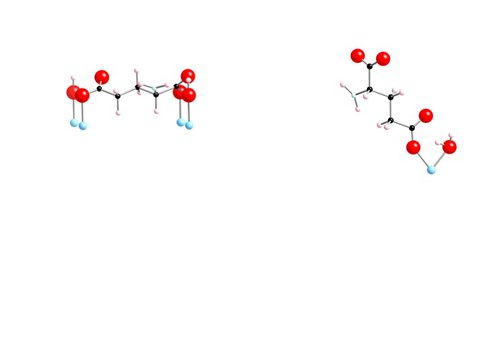
Interactions between aqueous amino acids and mineral surfaces influence the bioavailability of amino acids in the environment, the viability of Ti implants in humans, and the role of mineral surfaces in the origin of life on Earth. Over the past year CoI’s Hazen and Sverjensky and colleagues studied the adsorption of L-glutamate on the surface of rutile (α-TiO2, pHPZC = 5.4) in NaCl solutions using potentiometric titrations and batch adsorption experiments over a wide range of pH, ligand-to-solid ratio and ionic strength as shown in Fig. 4.1. We find that between pH 3 and 5 glutamate adsorbs strongly, up to 1.4 μmol m-2, and the adsorption decreases with increasing ionic strength. Potentiometric titration measurements of proton consumption for the combined glutamate-rutile-aqueous solution system show a strong dependence on glutamate concentration as shown in Fig. 4.2, where an extended triple-layer surface complexation model of all the experimental results required at least two reaction stoichiometries for glutamate adsorption, indicating the possible existence of at least two surface glutamate complexes. A possible mode of glutamate attachment involves a bridging-bidentate species binding through both carboxyl groups as shown in Fig. 4.3a, where this species can be thought of as “lying down” on the surface (as found previously for amorphous titanium dioxide and hydrous ferric oxide). A second mode of glutamate attachment involves a chelating species which binds only through the γ-carboxyl group as depicted in Fig. 4.3b: where this species can be thought of as “standing up” at the surface. The calculated proportions of these two surface glutamate species vary strongly, particularly with pH, ionic strength and glutamate concentration as shown in Fig. 4.4: where the calculated proportions of the two glutamate surface species as functions of pH at two different ionic strengths and glutamate loadings.
These results serve as a basis for a better quantitative understanding of how and under what conditions acidic amino acids bind to oxide mineral surfaces. For example, when glutamate is bound predominantly as the chelating species shown above, the a-carboxylate and amine groups might be free to interact above the surface with the free ends of adjacent glutamates, suggesting a possible mechanism for chiral self-organization and peptide bond formation. Based on our results we will be able to predict the environmental conditions most favorable for these interactions to occur. The results of this research were presented at the American Chemical Society Annual Spring Meeting.
Figure 4.1 Potentiometric Titration Measurements as a function of experimental parameters. Figure 4.1 – Adsorption of glutamate on rutile varies systematically as a function of pH, ligand to solid ratio, and salinity.
Figure 4.2: Proton consumption vs Glutamate concentration. Figure 4.2 – Potentiometric titration measurements of proton consumption for the combined glutamate-rutile aqueous suspension show a strong dependence on glutamate concentration
Figure 4.3: Potential Orientation of Glutamate on Rutile Surface. Figure 4.3 – Using an extended triple-layer surface complexation model we find that the experimental results are consistent with two different reaction stoichiometries for glutamate adsorption. A bridging bidentate species binding through both carboxyl groups and a “lying down” species that binds through the gamma caroboxyl group
Publications
-
Cleaves, H. J., Jonsson, C. M., Jonsson, C. L., Sverjensky, D. A., & Hazen, R. M. (2010). Adsorption of Nucleic Acid Components on Rutile (TiO 2 ) Surfaces. Astrobiology, 10(3), 311–323. doi:10.1089/ast.2009.0397
-
Jonsson, C. M., Jonsson, C. L., Estrada, C., Sverjensky, D. A., Cleaves, H. J., & Hazen, R. M. (2010). Adsorption of l-aspartate to rutile (α-TiO2): Experimental and theoretical surface complexation studies. Geochimica et Cosmochimica Acta, 74(8), 2356–2367. doi:10.1016/j.gca.2010.01.003
-
Jonsson, C. M., Jonsson, C. L., Sverjensky, D. A., Cleaves, H. J., & Hazen, R. M. (2009). Attachment of l -Glutamate to Rutile (α-TiO 2 ): A Potentiometric, Adsorption, and Surface Complexation Study. Langmuir, 25(20), 12127–12135. doi:10.1021/la901635t
- Cleaves, H.J., Jonsson, C.M., Jonsson, C.L., Sverjensky, D.A. & Hazen, R.M. (2009). Adsorption of nucleic acid components on rutile (TiO2) surfaces. 238th American Chemical Society National Meeting. Washington, DC.
- Cleaves, H.J., Sverjensky, D.A., Jonsson, C.M., Jonsson, C.L. & Hazen, R.M. (2008). Distinguishing multiple surface species of glutamate on hydrous ferric oxide. American Geophysical Union National Meeting. San Francisco.
- Estrada, C.F., Jonsson, C.M., Jonsson, C.L., Parikh, S.J., Sverjensky, D.A., Cleaves, H.J. & Hazen, R.M. (2008). Surface speciation of glutamate and aspartate on titanium dioxide. American Geophysical Union National Meeting. San Francisco.
- Ewing, R.C., Hazen, R.M. & Sverjensky, D.A. (2008). Mineral evolution of uranium and thorium. American Geophysical Union National Meeting. San Francisco.
- Hazen, R.M. (2009). Chapter 10 – Mineral Surfaces, Geochemical Complexities, and the Origins of Life. In: Deamer, D.W. & Szostak, J.W. (Eds.). Origins of Cellular Life. Cold Springs Harbor Perspectives.
- Hazen, R.M. (2009). Emergence and the experimental pursuit of the origin of life. In: Bertka, C.M. (Eds.). Exploring the Origins, Extent, and Future of Life: Philosophical, Ethical, and Theological Perspectives. New York: Cambridge University Press.
- Hazen, R.M. (2009). The geochemical origin of life. In: Knoll, A.H., Canfield, D.E. & Konhauser, K.O. (Eds.). A Geobiology Reader.
- Hazen, R.M., Jonsson, C.M., Jonsson, C.L., Cleaves, H.J. & Sverjensky, D.A. (2009). Molecule-mineral interactions and the origins of life. 238th American Chemical Society National Meeting. Washington, DC.
- Jonsson, C.M., Jonsson, C.L., Parikh, S.J., Sverjensky, D.A., Cleaves, H.J. & Hazen, R.M. (2008). Surface speciation of glutamate and aspartate on titanium dioxide. American Geophysical Union National Meeting. San Francisco.
- Jonsson, C.M., Jonsson, C.L., Sverjensky, D.A. & Hazen, R.M. (2008). The adsorption of aspartic acid on rutile: implications for biochirality. American Geophysical Union National Meeting. San Francisco.
- Jonsson, C.M., Jonsson, C.L., Sverjensky, D.A., Cleaves, H.J. & Hazen, R.M. (2009). Surface speciation of aspartate and glutamate on titanium dioxide. 237th American Chemical Society National Meeting. Salt Lake City.
- Jonsson, C.M., Jonsson, C.L., Sverjensky, D.A., Cleaves, H.J. & Hazen, R.M. (2009). Surface speciation of glutamate and aspartate on rutile as a function of environmental conditions. 238th American Chemical Society National Meeting. Washington, DC.
- Sverjensky, D.A. & Hazen, R.M. (2008). MoS2 and ReS2 stabilities as indicators of oxidation state on the early Earth. American Geophysical Union National Meeting. San Francisco.
- Sverjensky, D.A., Jonsson, C.M., Jonsson, C.L., Cleaves, H.J. & Hazen, R.M. (2008). Glutamate surface speciation on amorphous titanium dioxide and hydrous ferric oxide. Environmental Science & Technology, 42: 6034-6039.
- Sverjensky, D.A., Jonsson, C.M., Jonsson, C.L., Hazen, R.M. & Cleaves, H.J. (2009). Surface complexation models of glutamate and aspartate on rutile. 238th American Chemical Society National Meeting. Washington, DC.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Jennifer Stern
Collaborator
Caroline Jonsson
Postdoc
Shohei Ohara
Postdoc
Nabil Boctor
Research Staff
Henderson Cleaves
Research Staff
Chrisopher Johnson
Research Staff
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules