2011 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
Task 3.4.2 Tholin Analysis Based on Selective Detection of Functional Groups
Project Summary
Titan organics comprise a very complex mixture of compounds. Several approaches are being developed that provide targeted detection of specific functional groups, such as nitriles, imines, primary amines, and carbon-carbon multiple bonds.
Project Progress
Titan organics comprise a very complex mixture of compounds. Co-Investigator Jack Beauchamp and Graduate Student Kathleen Upton are developing several approaches that provide targeted detection of specific functional groups, such as nitriles, imines, primary amines, and carbon-carbon multiple bonds. Experiments for the selective and sensitive detection of primary amines through mass spectrometry are currently being conducted due to the biological prevalence of small molecule (<200Da) primary amines. The use of 18-Crown-6 ether adduct formation in electrospray ionization mass spectrometry (ESI/MS) has shown promise. Alternate oxygens of the 18-Crown-6 ether form 3 strong hydrogen bonds to a protonated primary amine, as illustrated in Figure 1.1
This allows for the selective detection of primary amine functionality in a complex mixture of organic compounds. As a test of this idea, laboratory produced tholins from two different plasma discharge generators (Figure 2) were complexed with 18-Crown-6. These include synthesis as described by Saker et. al. (2003) and the generator most recently developed by Mark Smith and coworkers.2,3
Each tholin was dissolved in dichloromethane at 2 mg/ml with 2mM 18-Crown-6 ether. Adduct formation with the Saker tholin is shown in Figure 3. The marked peaks in this figure, with M = 18-Crown-6 ether, correspond to two different series (triangles: NH2-(CH2)n-CH2CN, where n=0 is the first in the series, and circles: NH2-(CH2)n-CH(CN)2 ,where n=2 is the first in the series). The results of a similar experiment with the Smith tholin are shown in Figure 4, where several of the crown ether adduct peaks are labeled with an asterisk. Several of these match peaks observed in Figure 3 and others differ by two to six amu. These deviations could indicate the presence of unsaturation (CC or CN multiple bonds) or a different extent of nitrogen incorporation in the Smith tholins.
Complexation with 18-Crown-6 ether leads to the identification of primary amine functionality in both Tholin mixtures. It is particularly noteworthy that the two different preparations yield a very different mixture of primary amines. Beauchamp and Upton are currently trying to understand the origin of these differences in greater detail in addition to starting with fresh preparations of the tholins.
Primary amine functionality can also be selectively detected using an amine reactive reagent with a permanent charge. This can be accomplished with the quaternary ammonium tag , which reacts with primary amines in basic conditions as indicated in Figure 5. The tag is prepared in acetonitrile and is moisture sensitive.4
They have tested the amine reactive tag illustrated in Figure 5 using a mixture of the neurotransmitters dopamine, serotonin, and norepinephrine, which are all important biological compounds with primary amine functionality. Solutions of 100uM of the neurotransmitter in 0.1M TRIS at a pH of 8.2 with 10ug of the quaternary ammonium tag were allowed to react for 1 hour, after which they were quenched with 0.1% formic acid, and then diluted for analysis. Mass spectrometric analysis using ESI provided excellent yields and greatly enhanced sensitivity for detection of all three neurotransmitters compared to ESI of the underivatized amines.
Unfortunately, due to tholin reactivity with water and acid, the buffer and quench used with the amine reactive tag could not be used to directly derivatize tholin samples. The procedure was modified to use acetonitrile as the solvent. Tholins dissolve well in acetonitrile, as does the amine reactive tag, and tholins are known to be slightly basic due to nitrogen content. 1mg/mL of the Smith tholin and 100uM quaternary ammonium tag were combined in acetonitrle and left at room temperature. Analysis was performed at various points over a two day period, during which no changes indicative of the derivatization were observed in the mass spectrum. Future studies will explore a wider range of reaction conditions to achieve enhanced yields of derivatized products.
Additional experiments in progress include the use of reactive desorption electrospray ionization (DESI)5 for targeted identification of primary amines, using both of the reagents described above. By using this technique, exposure of the tholin to buffers or acids is minimized. The successful 18-Crown-6 adduct formation described earlier is being used as a starting point for reactive DESI, using neurotransmitters as test compounds. Once the ideal reactive DESI conditions are determined, the quaternary ammonium tag methodology will be attempted on neurotransmitters to determine any changes required in the experimental procedure. If successful they will again test the tholin samples to see if primary amines can be selectively detected.
References:
1. Julian R. R.; Beauchamp J. L. Int. J. Mass Spectrom. 2001, 210, 613-623.
2. N. Sarker, A. Somogyi, J. I. Lunine, and M. A. Smith, Astrobiology, 2003, 3(4), 719- 726.
3. A. Somogyi, C. Oh, M. A. Smith, J. I. Lunine, J. Am. Soc. Mass Spectrom., Vol. 16, 6, 2005, 850-859.
4. F. Che, L. D. Fricker, J. Mass Spectrom. 2005, 40, 238–249
5. I. Cotte-Rodríguez, Z. Takáts, N. Talaty, H. Chen, R. G. Cooks, Anal. Chem., 2005, 77 (21), 6755–6764
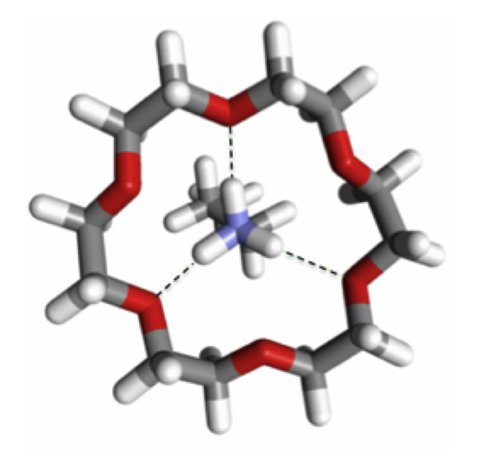 Figure 1. 18-Crown-6 ether complexed to protonated butylamine. The complex sequesters the proton at the primary amine site, preventing deprotonation during electrospray ionization.
Figure 1. 18-Crown-6 ether complexed to protonated butylamine. The complex sequesters the proton at the primary amine site, preventing deprotonation during electrospray ionization.
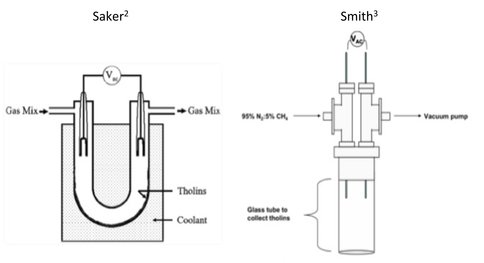 Figure 2. Tholin generators used to produce the two samples examined for primary amine content in this study. The Saker generator uses at gas flow system at atmospheric pressure and collects tholins below the discharge, while the Smith generator operates gas flow at low pressure and collects around the discharge
Figure 2. Tholin generators used to produce the two samples examined for primary amine content in this study. The Saker generator uses at gas flow system at atmospheric pressure and collects tholins below the discharge, while the Smith generator operates gas flow at low pressure and collects around the discharge
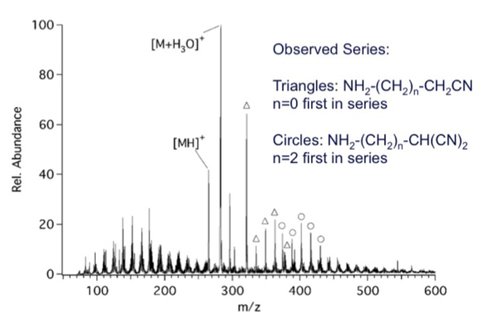 Figure 3. ESI/MS spectrum of 18-Crown-6 complexed Saker tholins. The triangles and circles indicated complexed species fitting a particular compound series.
Figure 3. ESI/MS spectrum of 18-Crown-6 complexed Saker tholins. The triangles and circles indicated complexed species fitting a particular compound series.
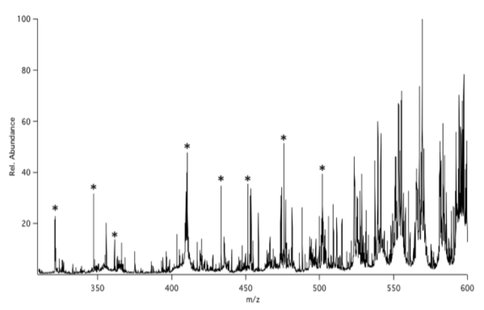 Figure 4. ESI/MS spectrum of 18-Crown-6 complexed Smith tholins. The asterisks indicate identified crown ether adducts.
Figure 4. ESI/MS spectrum of 18-Crown-6 complexed Smith tholins. The asterisks indicate identified crown ether adducts.
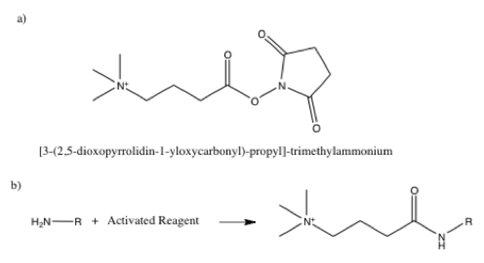 Figure 5. a) Amine reactive tag for derivatizing primary amines with a permanent charge. b) Derivatization of an arbitrary primary amine RNH2.
Figure 5. a) Amine reactive tag for derivatizing primary amines with a permanent charge. b) Derivatization of an arbitrary primary amine RNH2.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Kathleen Upton
Graduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
