2009 Annual Science Report
 University of Wisconsin
Reporting | JUL 2008 – AUG 2009
University of Wisconsin
Reporting | JUL 2008 – AUG 2009
Extra-Cellular Polymeric Substances as Armor Against Cell Membrane Rupture on Mineral Surfaces
Project Summary
Our interdisciplinary project examined the hypotheses that bacterial cell membranes are ruptured in contact with specific mineral surfaces, and that biofilm-forming extra-cellular polymeric substances (EPS) may have evolved to shield against membrane rupture (cell lysis). Furthermore, we proposed that mineral reactivity towards membranolysis should depend on its surface properties such as charge, reactive area, or free radicals generated by radiation and impacts on early Earth, Mars, and other worlds. The effect of EPS on preservation in the rock record will also be examined. By understanding the mechanisms for membranolysis, especially under the extreme conditions of high radiation and heavy impacts during early planetary history, the project addresses the NASA Astrobiology Institute’s (NAI) Roadmap goals of understanding the origins of cellularity, the evolution of mechanisms for survival at environmental limits, and preservation of biosignatures, and NASA’s Strategic Goal of advancing scientific knowledge of the origin and evolution of the Earth’s biosphere and the potential for life elsewhere.
Project Progress
We used vesicles of zwitterionic phospholipids, dipalmitoylphosphatidylcholine and ditridecanoylphosphatidycholine (DTPC), to represent prebiotic protocols (although we recognize that phospholipids are biologically produced, and that simpler lipids would have existed pre-biotically). We measured quantitative adsorption isotherms of both lipids on oxide particles (Figure 1) and imaged lipid bilayer formation on corresponding planar oxides using Atomic Force Microscopy (Figure 2). Results for both types of experiments at neutral pH revealed that adsorption is, indeed, oxide-dependent, with the affinity decreasing as corundum (α-Al2O3) > rutile (α-TiO2) > quartz (α -SiO2). This follows the sequence of decreasing points of zero charge of the oxides. We developed a model to explain the observed affinity sequence, which relies on a balance of electrostatic and van der Waals forces. The implication is that protocells would have had a greater affinity for adsorption on positively-charged surfaces than for negatively-charged surfaces.
We used whole bacterial cells to address the question of whether EPS provides armor against cell lysis. A newly developed EPS-lacking mutant strain of Pseudomonas aeruginosa (Figure 3), and the wild-type, which is a robust EPS producer (Figure 3), were used to conduct cell viability studies in suspensions of oxide particles with cells. Wild type cell viability was greater than that of the mutant type. Furthermore, viability was oxide-dependent, increasing as γ-Al2O3 < anatase (β-TiO2) < amorphous silica. Thus, our results confirm our hypothesis that EPS acts as a shield against the toxicity of certain minerals. In ongoing experiments, we are examining which surface properties of the minerals control their toxicity and the mechanisms of toxicity.
We are currently planning to conduct silicification experiments for both wild and mutant type cells in order to examine differences in morphology for preservation differences.
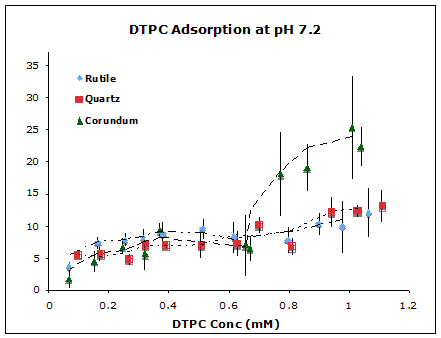
Figure 1. Adsorption isotherms of DTPC on corundum, rutile, and quartz particle suspensions at pH 7.2 and ionic strength 17 mM NaCl at 25 oC.
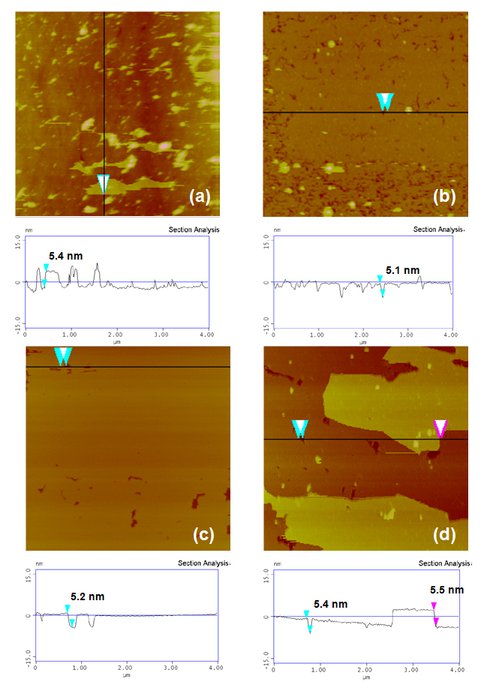
Figure 2. AFM Images of DTPC Bilayers On Planar, Single-Crystal Substrates. Plan view images (4 × 4 μm2) and vertical section profiles of DTPC bilayers adsorbed on planar surfaces of fused quartz (amorphous silica glass) (a), rutile (100) (b), mica (001) (c) and corundum (100) (d). Solution conditions: 1mM DTPC, 17 mM NaCl, 25 oC. Each bilayer height is ~ 5 nm. Note less than one or one bilayer on negatively0charged surfaces (a)-(c) and almost two complete bilayes on positively-charged surface (d).
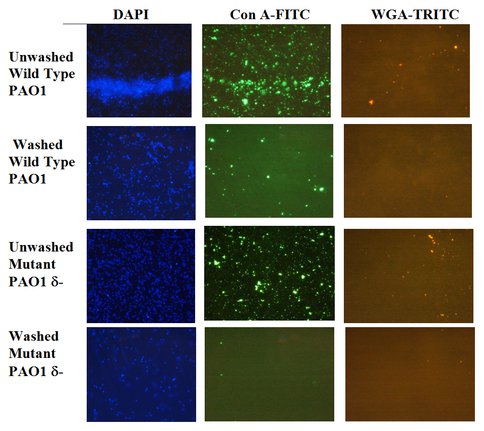
Figure 3. Visualization of Extracellular Polymeric Substances (EPS) Using Fluorescent Stains. Lectin stains for identifying EPS (1000x magnification) of washed and unwashed wild type and mutant type cells of Pseudmonas aeruginosa. Washing removes traces of EPS associated with the cells. DAPI (blue) stains for DNA indicating presence off cells (left); green con A-FTIC (center) and red WGA-TRITC (right) stain for specific EPS (Xu and Sahai, unpublished results).
Publications
-
Xu, J., Stevens, M. J., Oleson, T. A., Last, J. A., & Sahai, N. (2009). Role of Oxide Surface Chemistry and Phospholipid Phase on Adsorption and Self-Assembly: Isotherms and Atomic Force Microscopy. The Journal of Physical Chemistry C, 113(6), 2187–2196. doi:10.1021/jp807680d
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
William Hickey
Co-Investigator
Martin Schoonen
Co-Investigator
Jie Xu
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.4
Origins of cellularity and protobiological systems
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 7.1
Biosignatures to be sought in Solar System materials